





|
|
Cytoskeleton, biopolymer networks & cell mechanics
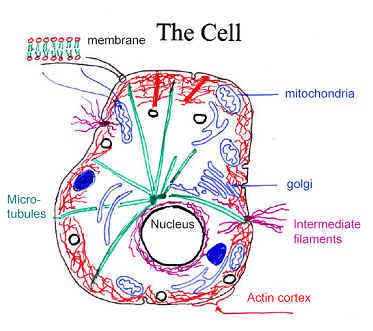 The structure and mechanical properties of plant and animal
cells depend in large part on a complex assembly of filamentous proteins and
associated binding proteins and enzymes. This cytoskeleton is not only
an important component of the cell but also a fascinating material in its own
right. One of the principal elements of the cytoskeleton is a network of
F-actin, a biopolymer consisting of two strands/necklaces of linked globular
proteins. These filaments are about 7 nm wide and can be many microns long.
Along with other cytoskeletal filaments such as microtubules, these are known as
semiflexible biopolymers because of their relative rigidity when
compared with most synthetic polymers. F-actin filaments and networks have been
extensively studied experimentally using in vitro constructs. Our
theoretical research aims at a quantitative basis with which to understand such
properties as the non-linear strain stiffening of cytoskeletal networks and
their highly tunable rigidity as a function of concentration and
binding/crosslinking proteins, and much of our work has been done in close
collaboration with experiment. The structure and mechanical properties of plant and animal
cells depend in large part on a complex assembly of filamentous proteins and
associated binding proteins and enzymes. This cytoskeleton is not only
an important component of the cell but also a fascinating material in its own
right. One of the principal elements of the cytoskeleton is a network of
F-actin, a biopolymer consisting of two strands/necklaces of linked globular
proteins. These filaments are about 7 nm wide and can be many microns long.
Along with other cytoskeletal filaments such as microtubules, these are known as
semiflexible biopolymers because of their relative rigidity when
compared with most synthetic polymers. F-actin filaments and networks have been
extensively studied experimentally using in vitro constructs. Our
theoretical research aims at a quantitative basis with which to understand such
properties as the non-linear strain stiffening of cytoskeletal networks and
their highly tunable rigidity as a function of concentration and
binding/crosslinking proteins, and much of our work has been done in close
collaboration with experiment.
|
A book chapter on models of the cytoskeleton:
Polymer-based models of
cytoskeletal mechanics, in Cytoskeletal Mechanics
(Cambridge Univ. Press, 2006) ed. by MRK Mofrad and RD Kamm
Other chapters can be found
here.
|
Our recent work:
A major focus of our recent work has been the non-equilibrium nature of the cytoskeleton, in which internal activity by enzymes generates stresses and motion that can domination Brownian motion. We study this by a combination of theoretical modeling and closes collaboration with our experimental colleagues, using both reconstituted in vitro model systems and living cells.
Recent highlights include:
D
Mizuno, C Tardin, CF Schmidt, FC MacKintosh,
Nonequilibrium mechanics of
active cytoskeletal networks.
Science, 315:370 (2007).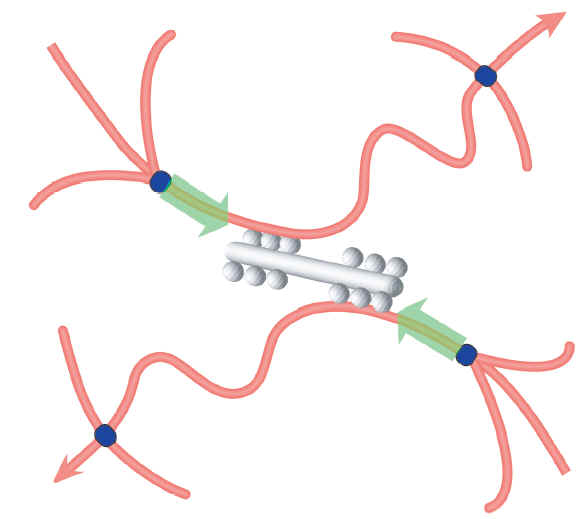
Abstract: Cells both actively generate and sensitively react to forces
through their mechanical framework, the cytoskeleton, which is a nonequilibrium
composite material including polymers and motor proteins. We measured the
dynamics and mechanical properties of a simple three-component model system
consisting of myosin II, actin filaments, and cross-linkers. In this system,
stresses arising from motor activity controlled the cytoskeletal network
mechanics, increasing stiffness by a factor of nearly 100 and qualitatively
changing the viscoelastic response of the network in an adenosine triphosphate–dependent
manner. We present a quantitative theoretical model connecting the large-scale
properties of this active gel to molecular force generation.
|
FC MacKintosh and AJ Levine
Non-equilibrium mechanics and dynamics of motor-activated gels
Physical Review Letters, 100:018104 (2008).
Abstract: The mechanics of cells is strongly affected by molecular motors that generate forces in the cellular cytoskeleton. We develop a model for cytoskeletal networks driven out of equilibrium by molecular motors exerting transient contractile stresses. Using this model we show how motor activity can dramatically increase the network’s bulk elastic moduli. We also show how motor binding kinetics naturally leads to enhanced low-frequency stress fluctuations that result in nonequilibrium diffusive motion within an elastic network, as seen in recent in vitro and in vivo experiments.
|
M Soares e Silva, M Depken, B Stuhrmann, M Korsten, FC MacKintosh, and GH Koenderink
Active multistage coarsening of actin networks driven by myosin motors
PNAS, 108: 9408 (2011).
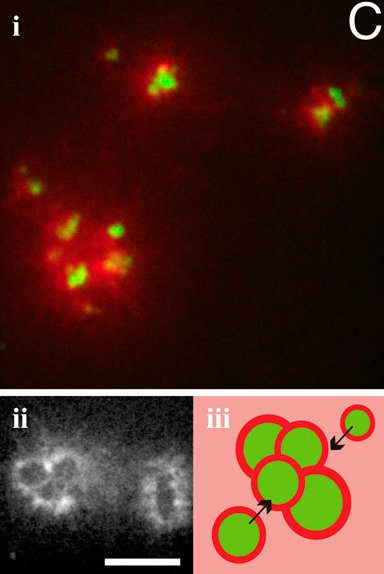 Abstract: In cells, many vital processes involve myosin-driven motility that Abstract: In cells, many vital processes involve myosin-driven motility that
actively remodels the actin cytoskeleton and changes cell shape.
Here we study how the collective action of myosin motors organizes
actin filaments into contractile structures in a simplified
model system devoid of biochemical regulation. We show that this
self-organization occurs through an active multistage coarsening
process. First, motors form dense foci by moving along the actin
network structure followed by coalescence. Then the foci accumulate
actin filaments in a shell around them. These actomyosin
condensates eventually cluster due to motor-driven coalescence.
We propose that the physical origin of this multistage aggregation
is the highly asymmetric load response of actin filaments: they can
support large tensions but buckle easily under piconewton compressive
loads. Because the motor-generated forces well exceed
this threshold, buckling is induced on the connected actin network
that resists motor-driven filament sliding.We show how this buckling
can give rise to the accumulation of actin shells around myosin
foci and subsequent coalescence of foci into superaggregates. This
new physical mechanism provides an explanation for the formation
and contractile dynamics of disordered condensed actomyosin
states observed in vivo.
|
CP Brangwynne, GH Koenderink, FC MacKintosh, DA Weitz
Cytoplasmic diffusion: molecular motors mix it up
J Cell Biology, 183: 583-587 (2008).
CP Brangwynne, GH Koenderink, FC MacKintosh, DA Weitz
Intracellular transport by active diffusion
Trends in Cell Biology, 19: 423 (2009).
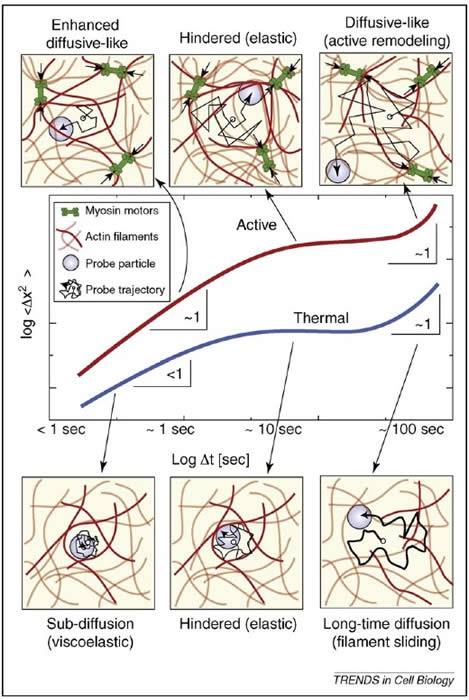
Abstract: All substances exhibit constant random motion at the microscopic scale. This is a direct consequence of ther- mal agitation, and leads to diffusion of molecules and small particles in a liquid. In addition to this nondirected motion, living cells also use active transport mechan- isms, such as motor activity and polymerization forces that depend on linear biopolymers and are therefore fundamentally directed in nature. Nevertheless, it has become increasingly clear that such active processes can also drive significant random fluctuations that can appear surprisingly like thermal diffusion of particles, but faster. Here, we discuss recent progress in quantify- ing this behavior and identifying its origins and con- sequences. We suggest that it represents an important and biologically tunable mechanism for trans- port in living cells.
|
CP Broedersz and FC MacKintosh
Molecular motors stiffen non-affine semiflexible polymer networks
Soft Matter, 7: 3186 (2011).
Abstract: Reconstituted filamentous actin networks with myosin motor proteins form active gels, in which motor
proteins generate forces that drive the network far from equilibrium. This motor activity can also
strongly affect the network elasticity; experiments have shown a dramatic stiffening in in vitro networks
with molecular motors. Here we study the effects of motor generated forces on the mechanics of
simulated 2D networks of athermal stiff filaments. We show how heterogeneous internal motor stresses
can lead to stiffening in networks that are governed by filament bending modes. The motors are
modeled as force dipoles that cause muscle like contractions. These contractions ‘‘pull out’’ the floppy
bending modes in the system, which induces a cross-over to a stiffer stretching dominated regime.
Through this mechanism, motors can lead to a nonlinear network response, even when the constituent
filaments are themselves purely linear. These results have implications for the mechanics of living cells
and suggest new design principles for active biomemetic materials with tunable mechanical properties.
|
Other related biophysics work
Gardel, ML, Shin,
JH, MacKintosh, FC, Mahadevan,
L, Matsudaira, P, Weitz, DA: Elastic Behavior of cross-linked and bundled
actin networks. Science, (2004). 304: 1301-1305.
(Link)
Head,
DA, Levine, AJ, and MacKintosh, FC: Deformation of crosslinked semiflexible
polymer networks. Physical Review Letters, (2003). 91: 108102.
(PDF)
Head,
DA, Levine, AJ, and MacKintosh, FC: Distinct regimes of elastic response and
deformation modes of cross-linked cytoskeletal and semiflexible polymer
networks, Physical Review E 68, 061907 (2003). (PDF)
JL
Harden, FC MacKintosh, and PD Olmsted, Budding and domain shape
transformations in mixed lipid films and bilayer membranes. Physical
Review E, (2005). 72: 011903. (PDF)
A
Montesi, M Pasquali and FC MacKintosh: Collapse
of a semiflexible polymer in poor solvent. Physical Review E, (2004).
69: 021916. (PDF)
Levine,
AJ, Liverpool, TB, and MacKintosh, FC: Dynamics
of rigid and flexible extended bodies in viscous films and membranes.
Physical Review Letters, (2004). 93: 038102. (PDF)
Ivanovska, IL, Pablo,
PJC, Ibarra, B, Sgalari, G, MacKintosh, FC, Carrascosa, JL, Schmidt, CF, and
Wuite, GJL: Bacteriophage
capsids: Tough nanoshells with complex elastic properties. Proceedings of
the National Academy of Sciences of the United States of America, (2004). 101:
7600-7605. |
| |
|

