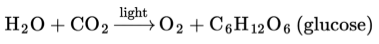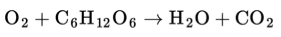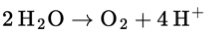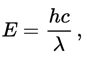Photosynthesis studies by Laser Remote Sensing (LRS) for high school students
Organisms like humans can use the energy stored in glucose for their energy supply. In the process carbon dioxide is released:
This is precisely the opposite reaction! Both kinds of organisms are dependent on each other for their energy supply. The waste of one is food for the other.
Photosynthesis is a complex process occurring in four steps:
- The plant absorbs sunlight.
- Energy from sunlight is used to excite an electron to a higher energy state.
- The electron enters a chain of electrons en is used to produce NADPH and ATP. These compounds are the fuel of living nature.
- Carbon atoms from carbon dioxide taken from the atmosphere are used to produce sugars. The plant uses ATP and NADPH molecules formed at step 3 to facilitate this formation of sugars.
Steps 1-3 are often referred to as the light reactions of photosynthesis because these reactions only occur when light shines on plants. Step 4 is referred to as the dark reaction, since it does not require sunlight. It should however be noted that a plant is also able to fixate carbon atoms from the atmosphere when exposed to light. To avoid confusion it is better to refer this reaction as the Calvin cycle, named after Melvin Calvin who received the 1961 Nobel Prize in chemistry for his contributions to research on this part of photosynthesis.
To obtain a better comprehension of the complex process called photosynthesis we will conduct a small research on how living plants use sunlight to produce sugars and oxygen.
The wavelength of light (color) determines the energy of photons:
where E is the photon energy, h is Planck's constant, c the speed of light and lambda is the wavelength of light.
Q2: What wavelength of light is required to split water?
In reality photons with a wavelength around 1000 nm do not have enough energy to start up the light reactions.
Q3: Should the light have a longer or shorter wavelength to start up the process?
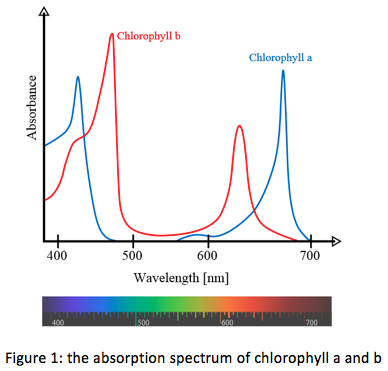
Figure 1 shows the absorption spectrum of chlorophyll. Plants have two types of chlorophyll: chlorophyll a en b. The absorption peaks of both these molecules lie at slightly different wavelengths. Therefore plants can absorb a range of colors of light, thereby making efficient use of sunlight.
Q4: Figure 1 shows that there is a rather wide range of wavelengths between 500 and 600 nm where light is not absorbed by either chlorophyll a or b. What are the colors of light in this range?
Q5: Can you explain why leaves are colored green?
Too much exposure to light damages a plant, just as it damages humans. When too much light is absorbed by a plant oxygen radicals are formed, which are extremely poisonous to a plant. Of course plants do not have the option to move out of the sun as humans have. However, plants do have several mechanisms to get rid of excess solar energy to protect themselves.
Electrons excited to high energy levels that are not used in the NADPH synthesis will decay to their ground state energy levels, releasing their energy by emitting photons (light) or heat. Solar energy absorbed by electrons can thus be used in three different ways:
- The energy of the excited chlorophylls is used to split water molecules, and the electrons and protons released in this process are used for the production of NADPH and ATP.
- The excited chlorophylls decay and the electrons return to their ground state levels through the release of heat.
- The excited chlorophylls decay and the electrons return to their ground state levels through the release of photons.
The third process is called fluorescence. Perhaps this is familiar from discotheque visits: tonic and bitter lemon contain quinine. Quinine absorbs ultraviolet light (also called black light), thereby exciting electrons to high energy level states. Upon decay to lower energy level states photons at visible wavelengths are emitted: drinks giving light!
Q6: Assume that light at a wavelength of 480 nm shines on a plant leaf. What is the color of the light and would a plant leaf be able to absorb it?
Q7: What is the energy of a photon with a wavelength of 410 nm (in eV)? Will this be sufficient to split water?
Q8: Assume that chlorophyll is excited by absorption of a 410 nm photon. It therefore gained 3 eV in energy. Upon decay to the ground state level a photon is emitted. Which colors the decaying chlorophyll cannot emit?
In the lab conducting a simple experiment you can demonstrate the fluorescence of chlorophyll. You will need a green plant with lots of chlorophyll (e.g. spinach) and an organic solvent, like acetone.
Requirements:
- Spinach or another plant with dark green leaves (dark green assures many chlorophylls).
- Test tube
- Acetone or methanol
- Filter paper (coffee filter works fine) and funnel
- Dark room
- UV light source (or multicolor LED light source)
Procedure:
- Grind the leaves (works better for frozen leaves)
- Dissolve it in 15 mL of acetone or methanol
- Run it through the funnel with the paper filter in it. Collect the solution in the test tube. This contains the chlorophyll.
- Take the test tube to a dark room and expose it to the UV light source. Decaying chlorophylls will emit visible light.
Photosynthesis lab
Photosynthesis is probably the most important process in living nature. Plants, algae and some bacteria use energy from sunlight and carbon dioxide taken from the air to produce sugars and other organic molecules. In doing so oxygen gets excreted. In the end, all organisms not able to use the sunlight's energy directly (including humans) are depending on photosynthesis for their energy supply. They depend on the sugars and oxygen that plants produce. Even is humans would only eat animals and fish, then still the energy gained from them would originate from plants through their food, and thus from photosynthesis.
The chemical reaction of photosynthesis can be summarized as:
Light absorption in plants
Plants absorb sunlight through their leaves. Leaves contain chloroplasts, which contain chlorophyll. Chlorophyll is able to extract energy from sunlight. Chlorophyll originates from Greek and is composed of two parts: chloros, which means green, and fyllon, which means leaf.
Chlorophyll contains clusters of proteins that can absorb light and extract energy from it. Plants and algea have two separate protein complexes that together complete the light reactions, called photosystem 1 and photosystem 2.
A chemical compound exists of protons, electrons and neutrons. Chemical bonds can pick up external energy in several ways, e.g. through heat or by absorption of electromagnetic radiation (light). Absorption of photons (energy) through sunlight results in electrons in their energetic ground state level to be excited to a higher energetic level. The energy of a photon determines the color or wavelength of the light, from violet (high energy) to red (low energy). When a photon with a specific wavelength is absorbed, an electron is excited to a higher energy state. The excess energy can be used to initiate chemical reactions. In plants the excess energy is used by chlorophyll to produce NADPH from NADP+. After this reaction chlorophyll is positively charged. After all it donated an electron to NADP+. Furthermore NADP+ is protonated. By splitting water simultaneously two protons and electrons are obtained and oxygen is formed and released to the atmosphere. The positively charged chlorophyll is neutralized and the remaining proton is used for the production of ATP.
Q1: Look up the standard electrode potential for the splitting of water: