![]()
Spin-orbit coupling
The spin-orbit coupling may be treated in a similar fashion as for one-electron atoms. The total angular momentum quantum number J = L + S will of course be used and proper eigenfunctions are those of L2, S2, J2 and Jz. We call these, which are the correct zero order functions,
![]()
The first order correction to the energy Es-o is then given by
![]()
J may take all integer values between L+S and |L-S| which means that the spin-orbit coupling splits a term into either 2L+1 or 2S+1 fine structure levels. Since the energy is independent of M, every level will have (2J+1)-fold degeneracy.
As for one-electron atoms, the spin-orbit coupling may be expressed in terms of the different angular momenta using
![]()
where A(L,S) is called the interval factor. The distance between adjacent fine structure levels J and J-1 is obtained as
![]()
This is the Landé interval rule.
 Use the result from first order perturbation theory
to determine the energy of the fine structure levels in 3P-state
from a p2-configuration.
Use the result from first order perturbation theory
to determine the energy of the fine structure levels in 3P-state
from a p2-configuration.
We have the following values of the quantum numbers: L=1, S=1, J=0,1,2
Inserting L and S gives the formula
![]()
with the following energies:
|
J |
|
|
2 |
A |
|
1 |
-A |
|
0 |
-2A |
 This result may be illustrated in a schematic extended energy level
diagram for the p2-configuration,
which incorporates the influence of spin-orbit
coupling. It looks like this.
This result may be illustrated in a schematic extended energy level
diagram for the p2-configuration,
which incorporates the influence of spin-orbit
coupling. It looks like this.
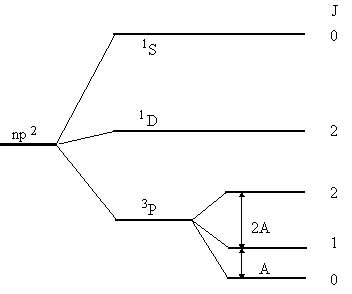
The spin-orbit splitting has been exaggerated in order to make a legible figure. Of course, spin-orbit splittings may be of this size for heavy atoms, but the perturbation treatment may then be less appropriate.
These energy levels are: E(3P0) = 0 eV; E(3P1) = 0,00203 eV; E(3P2) = 0,00539 eV;E(1D2) = 1,26386 eV; E(1S0) = 2,68406eV;
 Last change: 21 February 2001
Last change: 21 February 2001