Slater's rules
In many electron systems the electron-electron interaction is complicated
and there have been many attempts to introduce simplified methods for approximate
calculations of different kinds. The most well known were introduced by
Slater who derived rules for determination of screening constants s, which
give an effective nuclear charge Ze that is experienced by each
electron in the atom where
The rules are the following:
|
Principle quantum number
|
l quantum number
|
Number of electrons in the shell
|
s
|
|
1
|
0
|
2
|
0,3
|
|
> 1
|
0,1
|
x,y,z
|
0,35x + 0,85y + 1,00z |
|
3
|
2
|
x,y
|
0,35xd + 1,00yd
|
x = number of other electrons in the same shell as the screened electron
y = number of electrons in the shell with principle quantum number n
- 1
z = number of electrons in shells with principle quantum number
 n
- 2
n
- 2
xd = number of 3d-electrons
yd = number of electrons with principle quantum number
 3
and l < 2
3
and l < 2
 Example Calculate the binding energy for a 1s electron in helium.
Example Calculate the binding energy for a 1s electron in helium.
We get Ze = Z - s = 2 - 0,3 = 1,7
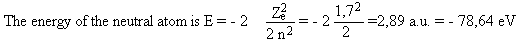
The energy of the ion is Eion = - 2 a.u. = - 54,42 eV
and the binding energy EB is obtained as the energy difference
EB = Eion - E = 78,64 - 54,42 = 24,22 eV
The experimental value is 24,587 eV.
 Exercise: Calculate the binding energy of the 3s electron in
the sodium atom and compare with the experimental value.
Exercise: Calculate the binding energy of the 3s electron in
the sodium atom and compare with the experimental value.
If we use the expression for the radius of the orbit obtained in the
Bohr model with the effective nuclear charge along with the principle quantum
number for the outermost electron we may estimate the atomic radius reff
from the formula
 Example Calculate the atomic radius for the neon atom.
Example Calculate the atomic radius for the neon atom.
We have s = 0,35x7 + 0,85x2 = 4,15
and thus Ze = 10 - 4,15 = 5,85.
Hence we get reff = 4/5,85x0,529167 = 0,362 Å
in reasonable agreement with the tabulated value, which is 0,354 Å.
 Exercise:
Exercise:
Calculate the atomic radius of an oxygen atom.
See a picture representing the
Atomic radii
and study the dependence on the position in the periodic Table.

Last change: 16 February 2001
![]() n
- 2
n
- 2 ![]() 3
and l < 2
3
and l < 2  Example Calculate the binding energy for a 1s electron in helium.
Example Calculate the binding energy for a 1s electron in helium.
