The aufbau principle.
The central field approximation and the Pauli principle constitute together the so-called aufbau principle (compare Atkins page 233). The lowest energy state of an atom is obtained by gradually filling shells from orbitals with lower to higher energy, i.e. in the order 1s, 2s, 2p, 3s, 3p, ..... The result is summarised by the so-called electron configuration where the number of electrons in each orbital is given as an upper index. Argon (Z=18), for example, has the ground state configuration
1s2 2s2 2p6 3s2 3p6
However, from Z=19 a certain change of this order starts to appear. The additional electron in this atom does not go into 3d, as could be expected, but instead into 4s. It is the comparatively strong penetration of the 4s electron that gives a lower energy for this electron than for 3d. Electrons in the 3d-shell first appear in scandium with Z=21. This deviation from the simplest principle remains throughout the periodic system. In fact, there is an empirical rule which can be used to determine the ground state configuration. It reads:
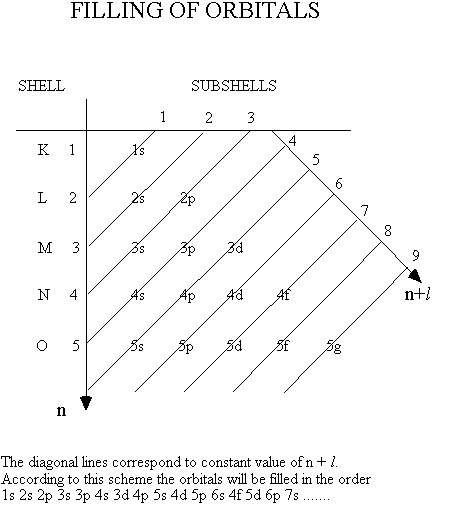
Shells are filled in order of increasing n+l and for a given value of n+l i in order of increasing n.
Except for the rare gases the procedure stops with an unfilled outer shell. For heavy atoms, the filling of the outer orbitals is often characterised by a certain disorder. Read more about this in Atkins section 9.8. The figure below show the schematic diagram for filling of subshells according to empirical rules.

 Example Give the electron configuration for the ground state
of the fluorine atom.
Example Give the electron configuration for the ground state
of the fluorine atom.
 Last change: 16 February 2001
Last change: 16 February 2001