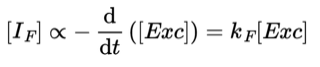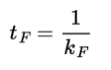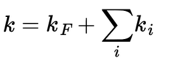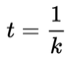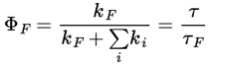Photosynthesis
Photosynthesis efficiently converts solar energy into a chemical form that can be used by living organisms. The primary processes of photosynthesis are the absorption of light by chromophores or pigments (= light absorbing molecules), subsequent energy transfer among many pigments and electron transfer, which finally results in proton translocation over a membrane. The photosynthetic membrane then acts like a biological battery, enabling to use the energy stored as a potential difference to create energy rich chemical bonds, which are used to drive life processes.
In figure 1.1 the reaction steps of this process are schematically drawn. The photosynthetic apparatus consists of a membrane-associated network of interconnected proteins that serve to hold the pigments in an ordered state. The different reaction steps are compartmentalised for regulation purposes and to ensure uni-directionality of the reactions. Light absorbing and energy transferring pigments are embedded in Light Harvesting Complexes (LHC's). When such a pigment absorbs a photon the excited state energy is transferred to a nearby pigment. Eventually such excitation reaches pigments of another type of photosynthetic complex: a Reaction Center (RC). Instead of further energy transfer, electrons are transferred within this complex from one side of the complex to the other side. These electrons are collected by carrier pigments, which also take up, and subsequently transport, protons. These can flow backwards mechanically whereby the energy is stored chemically.
Figure 1.1: Simplified diagram of the photosynthetic process. The different functional complexes are represented as blocks within a membrane. These complexes consist of many proteins and pigments. Energy transfer routes are shown as blue arrows, electron transfer as red arrows, proton transfer as green arrows and chemical fixation as a black arrow.
The chromophores of the plant RC's and LHC's are chlorophylls or chlorophyll-like pigments and carotenoids. By using different types of chlorophylls and carotenoids a wider window of absorption is created and energy and electrons can be transferred in an organised fashion. The most prominent pigments in plant photosynthesis are the chlorophyll's a and b and b-carotene. In figure 1.2 the structure and absorption spectrum of the most abundant LHC in plant photosynthesis is shown: the LHC2 complex. Roughly, the absorption spectrum is a sum of those of the constituting pigments.
Chlorophyll a has absorption maxima around 670 and 430 nm and chlorophyll b at 650 and 470 nm. The absorption spectrum of the carotenoids overlaps with that of the chlorophyll's in the blue/UV part starting at 500 nm.
Figure 1.2: Left: a structural model of the LHC2. The protein is depicted in dark grey and the 2 carotenoids (crossed in the center of the complex) as light grey. Chlorophyll's a are blue and chlorophyll's b yellow. Right: Absorption spectrum of the LHC2 complex at room temperature. (figure used with permission from dr. J. Salverda, thesis Vrije Universiteit).
In plants there are two clusters of RC's and LHC's, who work in series in order to maximise the energy conversion of incoming photons into energy rich chemical bonds. These clusters are called photosystems and are named photosystem 1 (PS1) and photosystem 2 (PS2). PS1 and PS2 consist of many antennae or light harvesting complexes (LHC's) which surround a few reaction centers (RC's). The above-depicted LHC2 complex for instance is mainly associated with the PS2 complex.
The two photosystems contain about 400 chlorophyll molecules; the vast majority of these belong to a LHC transferring their excitation to the RC's.
For all plants, the yield of photosynthesis can approach the theoretical maximum value, even under field conditions. However, because of fluctuations in light intensity in environmental conditions, photosynthesis frequently occurs at rates much below the maximum capacity provided by the available light, and under such conditions excess light has the potential to severely and irreversibly damage the pigment-protein complexes, leading to cell death. Therefore plants have evolved various photoprotective mechanisms, one of which actually converts excess absorbed radiation into heat, which can be harmlessly dispersed.
Interaction of light with biological matter
Matter can interact with an incoming lightbeam in various manners: light can be defracted, scattered and absorbed. Here we will deal with the absorption of light and the reactions, which follow absorption.
Biological molecules have a high tendency to absorb UV radiation. The amino- and nucleic acids of respectively proteins and DNA show distinct transitions in the near UV (200-300 nm). Many proteins include groups other than amino acids: chromophores. These are often, but not always covalently linked to a polypeptide chain and absorb in the visible region: 400-750 nm. Examples are flavins, iron porphyrins, rhodopsin and chlorins. The latter group contains the chlorophyll molecules, which are the dominant chromophores, or pigments in photosynthesis.
The absorption spectra of biological chromophores are considerably broader than the theoretically calculated homogeneously or lifetime broadened linewidth, which is only dependent on the excited state lifetime of the molecule. The reason for this is first of all the occurrence of vibronic transitions accompanying an electronic absorption. Depending on temperature this leads to a number of variations of transitions for every single chromophore. The other reason is inhomogeneous broadening which depend on the differences in local environment of every chromophore, which will influence both the electronic and vibronic transitions. When measuring the absorption of a bulk sample every chromophore will thus have a slightly different transition- and vibrational energy, leading to broadened spectra.
Photosynthesis starts off with the absorption of a photon by a chlorophyll or carotenoid molecule, which will be in the singlet-excited state (total spin S=0). An excited state is always unstable and will eventually return to the ground state. The ways by which excitation energy can be lost are: spontaneous and stimulated emission, internal conversion, decay to a triplet state (total spin S=1), energy transfer and photochemistry. It is clear that for efficient photosynthesis the latter two processes should be highly favourable over the others.
From all the processes leading to loss of excited states, the emission of light is the most easily detectable parameter.
Fluorescence
The emission of light from an excited electronic state is generally referred to as fluorescence. When the fluorescent molecule is the same as the absorbing molecule the lineshape of the fluorescence spectrum is expected to be a mirror image of the absorption spectrum because the same electronic and vibrational transitions are being probed. However, after excitation the molecule looses some energy by internal vibrations. Therefore the fluorescence spectrum is shifted to lower energy than the absorption spectrum. The fluorescence characteristics are also strongly dependent on the solvent. Since the excited state interacts often differently with the solvent than the ground state, more spectral shifts can be induced. This can also cause the fluorescence linewidth to be broader than the absorption linewidth because of an increased heterogeneity in the excited state. Because fluorescence occurs at lower frequency than that of the incident light it can easily be detected since there is no background signal (scatter) from the excitation source.
Therefore it is often possible to record fluorescence at concentrations two orders of magnitude lower than required for recording absorption spectra.
If a sample is illuminated to produce fluorescent excited states and the light is switched off the fluorescence decays. This decay is generally first order and the intensity of fluorescence [ IF ] would be characterised by the following rate law:
where [Exc] is the concentration of fluorescent states
The decay would be exponential with time with a rate constant kF. The inverse of this rate constant is called the radiative lifetime tF:
Thus, the radiative lifetime would be the lifetime of the emission if there would be no other decay possibilities of the excited state.
Note that the lifetime refers to a bulk property that measures how long an average molecule exists in a particular state.
In practice, however, fluorescence is just one of the ways by which the excited state returns to the ground state. The overall rate constant (k) for the depopulation of the excited state is obtained by summing the individual rate constants for all the competing processes (which are assumed to be simple processes like in eq. (1):
in which ki represents the various competing radiationless decay processes of the excited state.
The overall lifetime of the excited state (t) is again the reciprocal of the overall rate constant:
The quantum yield of fluorescence is the fraction of absorbed photons that lead to fluorescence. This is the number of photons fluoresced divided by the number absorbed. This again equals the ratio of the rate of fluorescence to the rate of absorbance. Since in a steady state the rate of absorbance equals the rate of decay of the excited state, the quantum yield can be described as:
Absolute values of the quantum yield are difficult to measure experimentally because instrument correction factors have to be known. In practice, they are obtained by comparison with a standard with known quantum yield.
Fluorescence of green plants
Fluorescence spectroscopy provides an excellent means to investigate photosynthetic processes in green plants. Spectrally the photosystems PS1 and PS2 can be distinguished since the PS1 antenna contains a number of chlorophylls that absorb at longer wavelength (less energy) than most of its other chlorophylls and of the chlorophylls embedded in the PS2 complex. Excitation energy in the PS1 antenna tends to focus on these special chlorophylls, which give rise to fluorescence at considerably longer wavelengths than those of PS2.
The normal circumstances for a plant is a low-light environment because most of its leaves will be in the shade of the few highest ones. In a so called dark-adapted plant the fluorescence quantum yield is very low. The main reason is that the usual decay channels of the singlet-excited state of chlorophyll (fluorescence, internal conversion into heat, intersystem crossing into a triplet state with total spin S=1) are circumvented by the energy and charge separation processes of photosynthesis.
When the amount of available light increases, however, the photosynthetic pathway becomes saturated and the plant reaches the maximal rate at which CO2 and H2O can be converted into O2 and organic matter. Under these conditions, the fluorescence yield increases, because the excited state of the chlorophylls can not decay anymore photosynthetically and the decay routes via the other processes (fluorescence, internal conversion, intersystem crossing) will increase.
With even more light, however, the fluorescence yield decreases again. The reason is that all decay channels become saturated including the occurrence of a singlet-to-triplet conversion of the excited state. These amounts of light are quite harmful for the plant, because the triplet state of chlorophyll can react with oxygen (which is a triplet in its ground state) to form singlet oxygen, an extremely reactive and hazardous species that will react with many components of the plant cell.
The deleterious effects of chlorophyll triplets are circumvented because of the presence of carotenoids, which not only serve to increase the absorption cross section but which also effectively can take over the triplet state of the chlorophylls. The energetics are such that a carotenoid triplet can not react with singlet oxygen. Since there are more chlorophyll's then carotenoids this quenching is limited to a certain amount of excited chlorophylls and thus to the light intensity.
Therefore all plants take extra precautions under high-light conditions by changing the antenna structure somewhat, with as final result a strongly increased decay by internal conversion. This prevents accumulation of triplet states, and has as a second effect that also the fluorescence yield decreases rather strongly.
The ability of a plant to show these types of fluorescence quenching depends on an intact photosynthetic apparatus; single chlorophylls will not show this kind of behaviour.
Thus, the fluorescence yield of green plants shows a number of dynamic variations that largely depend on the specific light conditions and on the capability of the plant to react on changes therein.
By means of LRS one increases the light intensity locally and in a controlled condition. In that way one can obtain information on the quality of the vegetation investigated.