

Fiber-optic
hydrogen
sensors
Introduction:
The
Hydrogen sensor project of VU University Amsterdam is
part of the "Sustainable
hydrogen program" of ACTS.
Goal
of the project
is the development of
sensitive, selective and fast indicator materials for optical fiber
hydrogen sensors. These
coatings will
be based on the "switchable" metal-hydride materials discovered
at
the
condensed matter group of the VU. These "switchable mirrors" are based
on materials involving alloys of
rare
earths, magnesium and some transition metals, whose optical proportions
depend
drastically on the amount of absorbed hydrogen. Fiber optics
as read-out is
selected,
as it
is intrinsically explosion-safe and easy to implement in a multi-sensor
safety system.
Switchable
mirrors:
Rare-earth
or transition metal based Mg alloys undergo a
transition from metal to semiconductor when hydrogen is absorbed in the
lattice. As a result the alloy changes optically from reflective to
transparent
or in some cases to a light absorbing black state.
|

|
|

|
|
A
mirror consisting of a Mg70Ti30
thin film layer |
|
After
applying a hydrogen
containing gas the mirror changes into a light absorbing Mg70Ti30Hx
layer. |
Our
goal is to develop a "metal-hydride switchable
mirror" which
is used as a safety detector in a future hydrogen economy.
|
 |
|
A
typical application for
(fiber-optic.) metal-hydride based sensors. Multiple
sensors could monitor optically the safety at several locations in a
hydrogen powered car. |
We
aim to
detect 10% of the lower explosion limit in air, (which is 4%) within
seconds
and with an optical change of a factor 10. The detector should allow
repeated
use.
Device
architecture:
Our
best
hydrogen detection material is Mg70Ti30.
This alloy forms
a strongly light absorbing metal-hydride when hydrogen exceeds the
equilibrium
hydrogenation pressure of 0.4 mbar. The ideal architecture of the
sensing layer
and its optical response is calculated from the dielectric constants of
the
used metals [1].
|
|
|
|
|
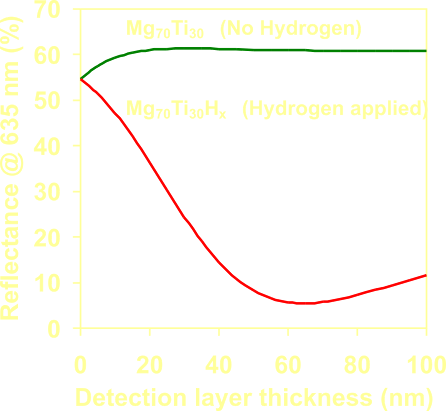
Calculated
effect of the layer
thickness on the optical response of the metal-hydride film (Mg70Ti30-Hx)
as a micro-mirror hydrogen detector. |
|
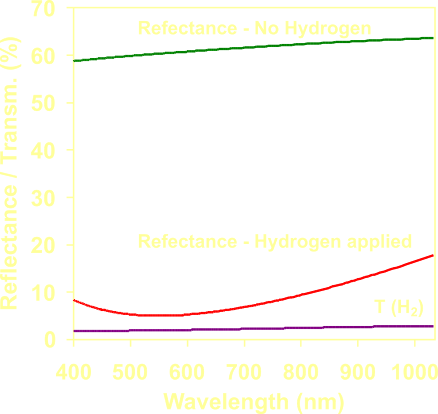
The
optical response vs
wavelength in the whole visible spectrum for a Mg70Ti30
layer of 60 nm, during hydrogen loading. |
The
Mg-Ti alloy
layer is covered by a 30 nm thick Pd layer which promotes the hydrogen
uptake
of the detector and prevents the layer from oxidation.
|
|
|
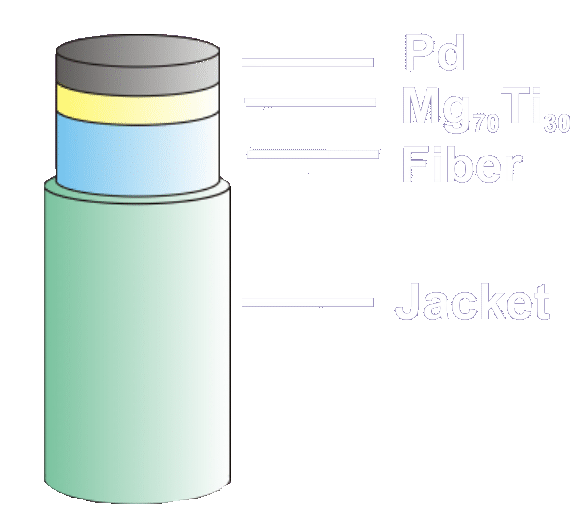
Typical
configuration of a fiber-optic micro-mirror hydrogen sensor,
based on a metal-hydride. The catalytic Pd layer can be
covered
with an extra protective coating. |
Device
preparation:
The
Mg-Ti
detection layer and the Pd cap layer are deposited on freshly cleaved
multi mode
glass fibers. By using glass fibers no electrical
leads are needed near the sensing point in a potentially explosive
environment. In a safety application multiple fiber detectors can be
read by a
single set of light source and detector. Deposition
of the layers is done by magnetron sputtering in argon. The composition
and
thickness are verified with a stylus profiler and Rutherford
Backscattering
Spectrometry.
|
 |
|
 |
|
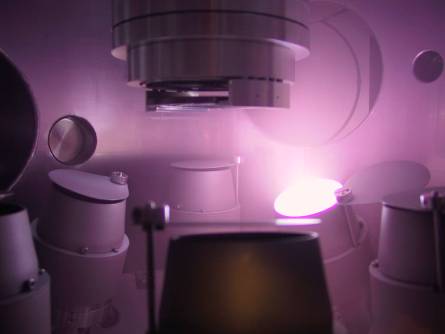
Argon-plasma magnetron
sputtering apparatus for depositing thin metal films on glass fibers
|
|
SEM image of a cleaved
end of a
glass fiber, after the deposition of the "switchable-mirror" layers.
|
Characterization:
We
connect the detector to a standard
bifurcating fiber which guides light from a white light source to the
indicator layer and guides the reflected light to a CCD
spectrometer. A low cost readout can be build from
a red LED, a CD-player beam splitter and a PIN diode.
|
 |
|
 |
|
Setup
for characterizing
the hydrogen detectors. Light is guided via a bifurcator to
the fiber end. The reflected light is measured by a CCD spectrometer.
The environment of the fiber end can be temperature controlled. |
|
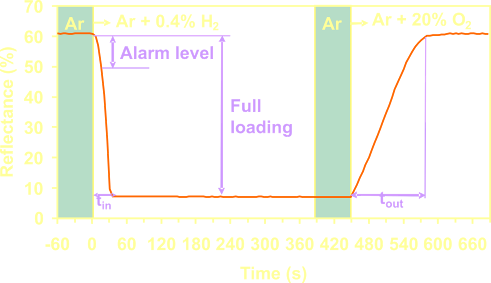
A
typical response of a Mg70Ti30
based detector. When a small concentration hydrogen is mixed
with the buffer gas (Argon) the reflection of the detector layer drops
by a factor 10 within seconds. |
The
detector
regenerates to its original state when the hydrogen
concentration drops below
the equilibrium pressure of the hydride. The unloading rate
of the detector increases when oxygen is present. The large
optical change upon hydrogen
loading allows the use of alarm levels in a hydrogen detector
application,
which improves the stability and reaction speed of the application.
|
 |
|
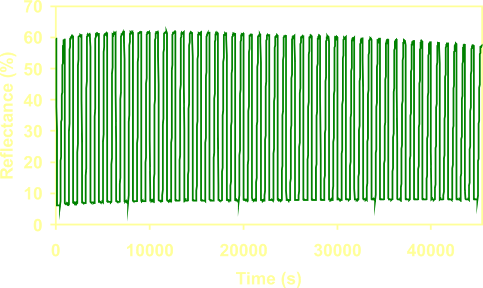
The
hydrogen detector
maintains its optical properties and reaction speed after many hydrogen
loading / unloading cycles. |
Over
100 stable cycles are measured, which
is more than enough for a
safety device. Oxidation of the detection layer due to cycling
stress can be reduced by using a thin NbOx
or AlOx
layer between the Pd
layer and the Mg70Ti30
layer.
Device improvements:
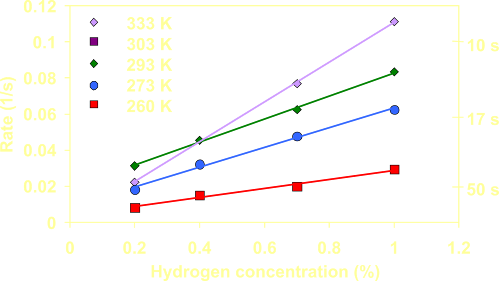
The
detection of low concentrations
hydrogen is within seconds.
The detector functions (unlike commercially available
detectors) well in oxygen poor
environments like argon glove boxes and over a large temperature range.
|
 |
|
The
hydrogen detector is
characterized for low concentrations hydrogen in a broad temperature
range; it still operates below the freezing point of water. |
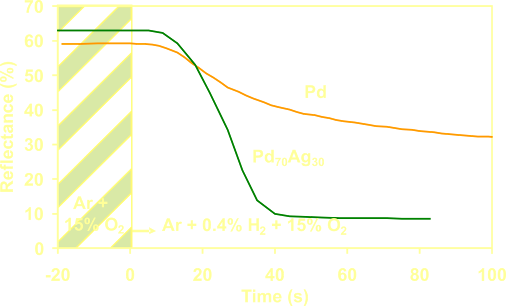
Oxygen
en carbon oxides in the environment of
the detector can lower the sensitivity and the kinetics due to
surface reactions on the catalytic Pd layer. These
effects are reduced by alloying the Pd layer with for example Ag [2]. A
hydrophobic organic coating on top of the Pd
layer prevents the detector from degradation by moist when
the detector is stored or used
for a long period in air.
|
 |
|
 |
|
By
alloying the catalytic
Palladium layer with Silver it becomes less sensitive for oxygen and
carbon molecules on the surface of the detector. |
|
The
left side of the
mirror is coated with a hydrophobic coating, which prevents the metal
from degradation by the moist in the air. |
Prototypes:
A
series of fiber optic hydrogen
detector prototypes
together with a
small readout system is currently spread among 10
hydrogen research laboratories in Europe. They
will test the
behaviour of the detector in a variety of conditions like in argon
glove
boxes or in polluted environments.
|
 |
|
 |
|
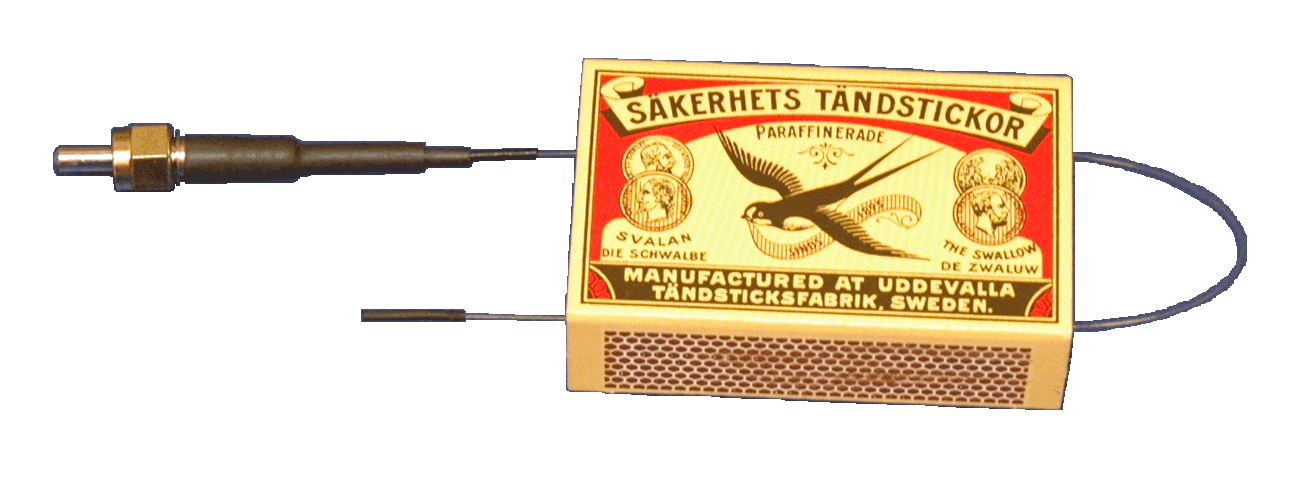
First
prototype of
the
fiber-optic hydrogen detector. |
|
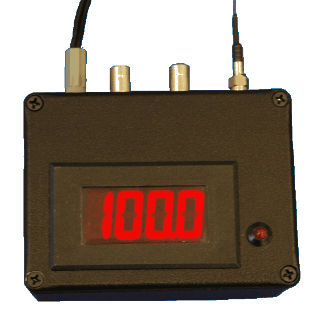
Readout
box for the field
test of the fiber-optic hydrogen detector. |
So
far we observe a reproducible
detection of pure hydrogen in air during a test period of several
months. Our
research will now focus on developing a sensor for quantitative reading
of the
hydrogen concentration in air.
|
 |
|
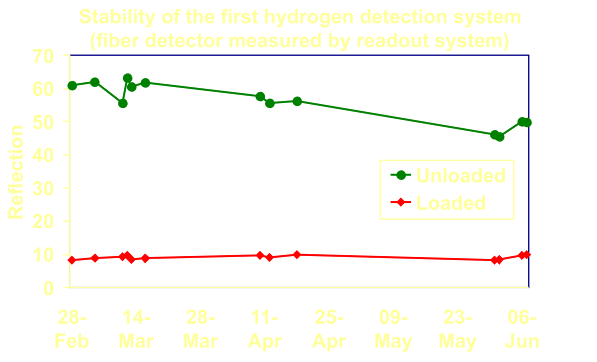
First
results of the metal-hydride based fiber-optic hydrogen detector field
test. The detector is tested for a period of months in air, several
times a small cloud of hydrogen was released near the fiber-end. The
drop in reflection in the unloaded state was caused by a drift in the
readout box. |
References:
[1].
M. Slaman, B. Dam, M. Pasturel, D.M. Borsa, H. Schreuders, J.H. Rector,
R. Griessen, Fiber
optic hydrogen
detectors containing Mg-based metal
hydrides, In press
by Sensors & Actuators B: chemical
123, Issue 1, 10 April 2007, Pages 538-545
[2]. M. Slaman, B. Dam,
H. Schreuders, R. Griessen, optimization
of Mg-based fiber optic
hydrogen detectors by alloying the catalyst,
Int. J. Hydrogen
energy 33
(2008) 1084-1089.
[3].
Patent: Protective
coating for metal-hydride based devices,
filed
in march 2006, Inventors: B. Dam, H. Schreuders, M. Slaman and M.
Pasturel (owned by ACTS); WO2007126313, NL1031708, (P6007119NL).
[4]. Patent: Optical
switching device, filed in
September 2005,
Inventors: B. Dam, R. Griessen, W. Lohstroh, M. Pasturel and
M. Slaman (owned by ACTS); WO2007049965, NL1030299, EP1952195,
CA2627651, AU2006306870, US2008291452, PCT/NL2006/050268 (P6003851NL).
Download A4
poster (.pdf) (900 KB)
Contact
Back


















